Transform Your Laboratory



At-a-Glance
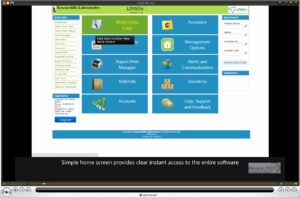
LimsDX is the premier Laboratory Information System for accessioning and reporting clinical Laboratory results. In one fully integrated, customizable LIS, you can:
- Manage the complex workflow of a Clinical Laboratory from sample receipt to final reporting and billing.
- Provide your External clients with a single Web-Enabled Portal to order, track and access all of their case information, and clinical test reports.
- Equip your laboratory with a suite of powerful analysis tools to organize assay runs, track samples, analyze data and report results.
- Provide office staff, technicians, and administrators with the tools they need to be more productive.
- Seamless integration into any esoteric testing laboratory

- HTML5 Web based interface. No software to install.
- Android and iPad enabled.
- Secure HTTPS encryption, and database encryption.
- Complete Audit Trail: LimsDx provides a secure, time-stamped audit trail exceeding 21 CFR part 11 requirements, capturing the identity of anyone who creates or modifies an electronic record, when the action occurred, and the changes made.
- Powerful user management security tools provide granular management to all LIMS functions.
- Easily create custom Tests from the web based interface. Simple and convenient creation of custom data fields. Ability to import files and images into a test.
- Create professional, informative reports using the integrated ‘Drag and Drop’ Report Builder.
- Minimize administration and maintenance costs. System server will run on linux or windows.
- Perform simple, automatic QA/QC analysis using sophisticated data management tools.
Key Features

Standard Workflow management
LimsDx has quick to navigate screens, intuitive search options and perceptive design allowing you to move quickly and accurately
- Clear and concise Workflows from Accession to Clinical Report.
- Comprehensive and Customizable Queries, and quality metrics.
- Granular User permissions.
- Completely Web enabled. Access by Computer, tablet or even phone.

Sample and Aliquot Management
- Track Sample status from one Lab department to the next, complete with Bar-code and Aliquot management functionalities.
- Track collected samples, allocate a single sample to multiple tests, and even aliquot or split a sample and track each aliquot individually.
- Generate Sample Barcodes, and auto-assign and auto-validate assay requirements to the samples received.

Access Management
- LimsDx is available on the cloud as a SAAS implementation or as a standalone product implemented within your facility.
- Secure web based usage and management.
- Optional Physician portal for ordering tests, viewing test progress and publishing clinical reports.
- Completely Web based administration. User, database, assay and clinical report creation and management is all enabled through the web based interface

Chain of Custody
- LimsDx allows you to create chain of custody flows, and decide what actions are tracked for each sample.
- You can generate custom reports filtered using date ranges, sample number and user-id, which is exportable to MS Excel.

Security and Audit Trail
- Granular user based access. Ability to restrict or allow user access on a page by page level.
- Audit trails ensure that LimsDx stores date and time stamped records of each action.
- Encryption of all HIPAA information within the database for added security.
- SSL encryption for all web based entry

Custom Alerts
- LimsDx Users can set up alerts for each other and ordering physicians in your Lab for task reminders.
- LimsDx comes integrated with Email and SMS functionality to send reminders, alerts and reports.
ResearchDx Divisions
Contract Services
- Full CRO Capabilities
- Biomarker Discovery
- Assay Development
- Regulatory Filings
- Project Management
Diagnostic Laboratory
- Assay Commercialization
- PreClinical GLP Services
- Clinical GCLP
- CLIA Testing Services
- Orthoginal Validations